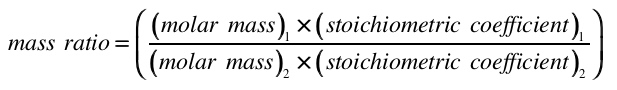Using Mass Ratios in Stoichiometric ProblemsIn this exercise, you will need to examine the chemical equation and construct a mass ratio relating the reactants and products. You will then multiply this ratio by the given mass of the reactant (or product) in order to find the required value. Remember that the mass ratio is the molar mass multiplied by the stoichiometric coefficient, for each reactant or product:
eMail your Instructor: When you have correctly answered five questions, you may enter your email addresses to receive credit for this assignment. |