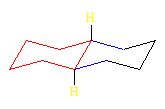
cis-Decalin
The hydrogens (gold) on the ring junction carbons are cis in this diastereomer. Both of the six-membered rings assume chair conformations. The carbons of one ring are attached to an axial and to an equatorial position on the other ring. In this model, the blue C is equatorial on the red ring, and the green C is axial on the red ring. A ring flip, which interconverts axial and equatorial positions, can occur.
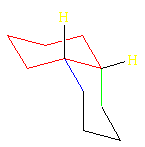
cis-Decalin Ring Flip
This is the ring flip of the structure above. Now the blue C is axial on the red ring and the green C is equatorial.
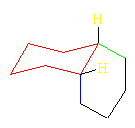
trans-Decalin
The hydrogens (gold) on the ring junction carbons are trans in this diastereomer. Both of the six-membered rings assume chair conformations. Both carbons of one ring are attached to equatorial positions of the other. This is true for both rings. In this model, both blue C's are bonded to equatorial positions on the red ring. trans-Decalin is rigid and cannot undergo a ring flip because this would convert all axial bonds to equatorial. One ring is not large enough to bridge axial positions on the other because they point in opposite directions. As was the case with the 1,2-dimethylcyclohexanes, trans-decalin, with both rings attached equatorially to the other ring, is more stable than cis-decalin by 2.7 kcal/mol (11.3 kJ/mol).