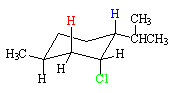
Menthyl Chloride
In the case of menthyl chloride, the conformation that has the chlorine (green) axial also has the methyl and isopropyl groups axial. This conformation is much less stable than its ring-flip conformation. As a result, the reaction has a higher activation energy and is relatively slow because some of the strain energy of the higher energy conformation is still present in the transition state.
Only the red hydrogen is anti to the chlorine, so the reaction produces a single alkene product.
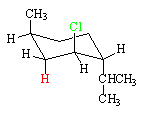
Neomenthyl Chloride
In the case of neomenthyl chloride, the conformation that has the chlorine (green) axial has the methyl and isopropyl groups equatorial. This conformation is more stable than its ring-flip conformation. As a result, E2 elimination is about 40 times faster for neomenthyl chloride than menthyl chloride because there is less steric strain in the transition state for neomenthyl chloride.
Both the blue hydrogen and the red hydrogen are anti to the chlorine, so the reaction produces two alkene products. In accord with Zaitsev's rule, the more highly substituted alkene,resulting from the loss of the blue hydrogen, is the major product.