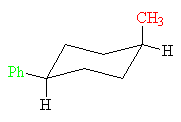
cis-1-Methyl-4-phenylcyclohexane
This conformation of the cis-isomer has an axial methyl group (red) and an equatorial phenyl group (green). It has 1.7 kcal/mol (7.1 kJ/mol) of strain energy.
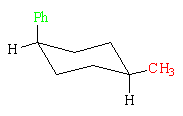
This conformation of the cis-isomer has an equatorial methyl group (red) and an axial phenyl group (green). It has 2.9 kcal/mol (12.1 kJ/mol) of strain energy. Because the axial destabilization energy for a phenyl group is larger than that for a methyl group, the first conformation is more stable than the second by 2.9 - 1.7 = 1.2 kcal/mol (5.0 kJ/mol).
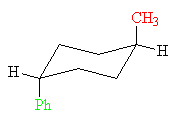
trans-1-Methyl-4-phenylcyclohexane
This conformation of the trans-isomer has both groups axial. It has a total strain energy of 1.7 + 2.9 = 4.6 kcal/mol (19.2 kJ/mol).
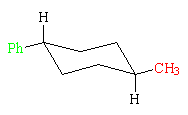
This conformation of the trans-isomer has both groups equatorial, so it has no strain energy. It is 4.6 kcal/mol (19.2 kJ/mol) more stable than the other conformation. To determine the relative stabilities of the trans-isomer and the cis-isomer, the strain energies of the more stable conformation of each are compared. Overall, the trans-isomer is more stable than the cis-isomer by the amount of strain due to the axial methyl group in the more stable conformation of the cis-isomer, 1.7 kcal/mol (7.1 kJ/mol).