menthol
all groups equatorial
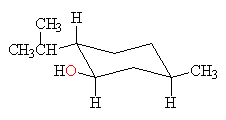
menthol
all groups axial
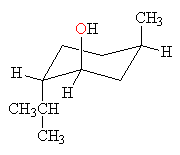
Menthol has all three groups equatorial in the conformation below on the left and all three groups axial in the conformation below on the right. The conformation on the left, with all groups equatorial, is much more stable.
mentholall groups equatorial  |
mentholall groups axial  |
Neomenthol differs from menthol in the configuration at the carbon attached to the hydroxy group. In the most stable conformation, on the left below, the methyl and isopropyl groups are equatorial and the hydroxy group is axial. In the less stable conformation on the right, the the methyl and isopropyl groups are axial and the hydroxy group is equatorial. Because menthol has the conformation with all of the groups equatorial, it is more stable by 0.9 kcal/mol (3.8 kJ/mol), the axial strain energy of the OH group.
neomentholMe and iPr equatorial, OH axial 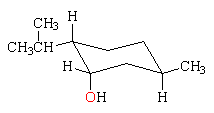 |
neomentholMe and iPr axial, OH equatorial 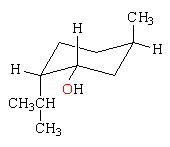 |