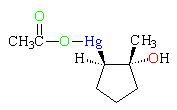
Mercurinium Ion
The mercury electrophile adds to the double bond in a process very similar to the formation of a bromonium ion. The species with a three-membered ring containing a positively charged Hg (blue) is termed a mercurinium ion. It is very similar to a bromonium ion or a protonated epoxide in its reactions. (The oxygens of the acetate group are green.)
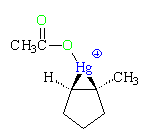
Water acts as a nucleophile and opens the ring of the mercurinium ion. As was the case for a bromonium ion and a protonated epoxide, the mechanism is borderline SN2, so the oxygen (red) attacks the more highly substituted carbon with inversion of configuration.