Molar StoichiometryIn this exercise, you will be given a balanced chemical equation and a molar quantity for a reactant or product. You will then be required to calculate a molar amount for a second reactant or product. In order to solve these problems, you will need to set up two molar ratios; a known ratio based on reaction stoichiometry and an unknown ratio based on the given quantity. For example, in the reaction shown below, if you were given a certain number of moles of A and asked to find moles of C, you would set up the ratio: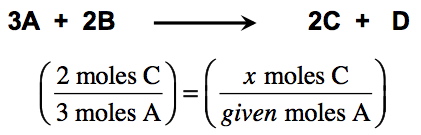
Use four significant figures; do not include units or attempt to use scientific notation. eMail your Instructor: When you have correctly answered five questions, you may enter your email addresses to receive credit for this assignment. |