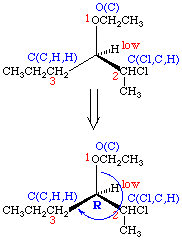
The rules for assignment of priorities in order to assign absolute configuration are based on the same set of rules which have been used previously for assigning E and Z stereochemistry. The procedure is fairly straightforward for simple compounds; first, you assign priorities to the groups attached around the chiral center. Next, you rotate the molecule so that the lowest priority group is pointing towards the back (away from you). Finally, you examine the remaining group priorities and determine if they are now arranged so that the priority decreases clockwise (R, for rectus) or counterclockwise (S, for sinister).
These rules are restated below, with examples:
- 1.
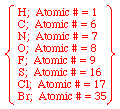
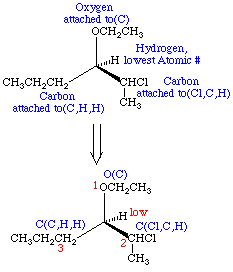
- Examine the four atoms directly attached to the chiral center in question. Assign priorities in order of decreasing atomic number; the atom with the highest atomic number is #1, the next is #2, etc.
- 2.
- If a decision regarding priority cannot be reached using Rule #1, compare the atomic numbers of the second atoms in each substituent, then the third, etc., until a difference is found.
- 3.
- Multiple bonds count twice (or three times) when examining substituents.
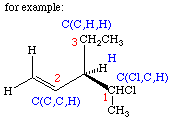
- 4.
- Once the priorities have been assigned, rotate the molecule in space so that the lowest priority group is pointing back. Connect the three remaining groups in order of decreasing priority and examine the direction of the resulting rotation. Rotation which is clockwise is termed R (rectus; right) and rotation which is counterclockwise is termed S (sinister; left).